Which of the Following Can Both Catalyze Chemical Reactions and Carry Information to Copy Itself?
To fully understand the processes occurring in present-day living cells, we need to consider how they arose in evolution. The most fundamental of all such issues is the expression of hereditary data, which today requires extraordinarily circuitous machinery and proceeds from Dna to protein through an RNA intermediate. How did this machinery arise? Ane view is that an RNA earth existed on Earth before modern cells arose (Figure 6-91). According to this hypothesis, RNA stored both genetic information and catalyzed the chemical reactions in archaic cells. Just later in evolutionary time did DNA have over as the genetic cloth and proteins get the major catalyst and structural component of cells. If this idea is correct, then the transition out of the RNA earth was never consummate; as we accept seen in this chapter, RNA however catalyzes several fundamental reactions in modern-day cells, which can be viewed every bit molecular fossils of an earlier world.

Effigy six-91
Time line for the universe, suggesting the early existence of an RNA earth of living systems.
In this department we outline some of the arguments in back up of the RNA earth hypothesis. We will come across that several of the more surprising features of modernistic-twenty-four hours cells, such as the ribosome and the pre-mRNA splicing machinery, are nigh easily explained by viewing them as descendants of a complex network of RNA-mediated interactions that dominated cell metabolism in the RNA earth. We likewise discuss how Deoxyribonucleic acid may have taken over as the genetic material, how the genetic code may have arisen, and how proteins may have eclipsed RNA to perform the majority of biochemical catalysis in modern-day cells.
Life Requires Autocatalysis
Information technology has been proposed that the offset "biological" molecules on Earth were formed past metal-based catalysis on the crystalline surfaces of minerals. In principle, an elaborate system of molecular synthesis and breakup (metabolism) could have existed on these surfaces long before the first cells arose. Just life requires molecules that possess a crucial property: the power to catalyze reactions that atomic number 82, directly or indirectly, to the production of more molecules similar themselves. Catalysts with this special self-promoting property tin use raw materials to reproduce themselves and thereby divert these aforementioned materials from the production of other substances. But what molecules could have had such autocatalytic properties in early cells? In present-day cells the most versatile catalysts are polypeptides, composed of many dissimilar amino acids with chemically diverse side chains and, consequently, able to adopt diverse 3-dimensional forms that bristle with reactive chemical groups. But, although polypeptides are versatile every bit catalysts, there is no known way in which one such molecule can reproduce itself by direct specifying the formation of another of precisely the aforementioned sequence.
Polynucleotides Can Both Store Information and Catalyze Chemical Reactions
Polynucleotides have i property that contrasts with those of polypeptides: they can directly guide the germination of exact copies of their own sequence. This chapters depends on complementary base pairing of nucleotide subunits, which enables one polynucleotide to human action as a template for the germination of some other. Equally nosotros have seen in this and the preceding chapter, such complementary templating mechanisms lie at the eye of DNA replication and transcription in modern-twenty-four hour period cells.
But the efficient synthesis of polynucleotides by such complementary templating mechanisms requires catalysts to promote the polymerization reaction: without catalysts, polymer formation is slow, error-prone, and inefficient. Today, template-based nucleotide polymerization is rapidly catalyzed by protein enzymes—such equally the Deoxyribonucleic acid and RNA polymerases. How could it be catalyzed before proteins with the appropriate enzymatic specificity existed? The beginnings of an answer to this question were obtained in 1982, when it was discovered that RNA molecules themselves tin can act as catalysts. We take seen in this chapter, for example, that a molecule of RNA is the catalyst for the peptidyl transferase reaction that takes place on the ribosome. The unique potential of RNA molecules to human activity both as information carrier and as catalyst forms the footing of the RNA globe hypothesis.
RNA therefore has all the properties required of a molecule that could catalyze its own synthesis (Figure 6-92). Although self-replicating systems of RNA molecules have not been establish in nature, scientists are hopeful that they can be constructed in the laboratory. While this demonstration would not bear witness that cocky-replicating RNA molecules were essential in the origin of life on World, it would certainly suggest that such a scenario is possible.
Figure 6-92
An RNA molecule that can catalyze its own synthesis. This hypothetical process would require catalysis of the production of both a 2nd RNA strand of complementary nucleotide sequence and the utilise of this second RNA molecule as a template to grade many (more than...)
A Pre-RNA Globe Probably Predates the RNA World
Although RNA seems well suited to form the basis for a self-replicating set of biochemical catalysts, it is unlikely that RNA was the showtime kind of molecule to exercise so. From a purely chemical standpoint, it is hard to imagine how long RNA molecules could be formed initially by purely nonenzymatic means. For one thing, the precursors of RNA, the ribonucleotides, are difficult to grade nonenzymatically. Moreover, the formation of RNA requires that a long series of 3′ to 5′ phosphodiester linkages course in the face of a gear up of competing reactions, including hydrolysis, 2′ to five′ linkages, 5′ to five′ linkages, and so on. Given these problems, it has been suggested that the starting time molecules to possess both catalytic action and information storage capabilities may have been polymers that resemble RNA just are chemically simpler (Figure 6-93). We do not take any remnants of these compounds in present-day cells, nor practice such compounds leave fossil records. Notwithstanding, the relative simplicity of these "RNA-like polymers" make them better candidates than RNA itself for the first biopolymers on Earth that had both information storage capacity and catalytic activity.
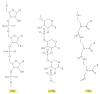
Figure 6-93
Structures of RNA and 2 related information-carrying polymers. In each case, B indicates the positions of purine and pyrimidine bases. The polymer p-RNA (pyranosyl-RNA) is RNA in which the furanose (five-membered ring) course of ribose has been replaced (more...)
The transition between the pre-RNA world and the RNA world would take occurred through the synthesis of RNA using ane of these simpler compounds equally both template and goad. The plausibility of this scheme is supported by laboratory experiments showing that one of these simpler forms (PNA—run into Effigy vi-93) can human action as a template for the synthesis of complementary RNA molecules, because the overall geometry of the bases is similar in the two molecules. Presumably, pre-RNA polymers besides catalyzed the formation of ribonucleotide precursors from simpler molecules. Once the beginning RNA molecules had been produced, they could have diversified gradually to accept over the functions originally carried out past the pre-RNA polymers, leading eventually to the postulated RNA world.
Single-stranded RNA Molecules Tin can Fold into Highly Elaborate Structures
We have seen that complementary base-pairing and other types of hydrogen bonds can occur between nucleotides in the aforementioned chain, causing an RNA molecule to fold upwards in a unique way adamant by its nucleotide sequence (run across, for example, Figures 6-6, half dozen-52, and vi-67). Comparisons of many RNA structures take revealed conserved motifs, brusque structural elements that are used over and over again every bit parts of larger structures. Some of these RNA secondary structural motifs are illustrated in Figure vi-94. In addition, a few common examples of more circuitous and often longer-range interactions, known equally RNA tertiary interactions, are shown in Effigy 6-95.

Figure vi-94
Common elements of RNA secondary structure. Conventional, complementary base-pairing interactions are indicated past red "rungs" in double-helical portions of the RNA.
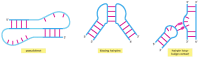
Figure six-95
Examples of RNA 3rd interactions. Some of these interactions tin can join distant parts of the aforementioned RNA molecule or bring ii separate RNA molecules together.
Protein catalysts require a surface with unique contours and chemical backdrop on which a given set of substrates can react (discussed in Chapter 3). In exactly the same way, an RNA molecule with an appropriately folded shape tin serve as an enzyme (Figure 6-96). Like some proteins, many of these ribozymes work by positioning metal ions at their active sites. This characteristic gives them a wider range of catalytic activities than can be accounted for solely by the limited chemical groups of the polynucleotide chain.

Figure 6-96
This elementary RNA molecule catalyzes the cleavage of a second RNA at a specific site. This ribozyme is found embedded in larger RNA genomes—called viroids—which infect plants. The cleavage, which occurs in nature at a distant location on (more...)
Relatively few catalytic RNAs be in modern-day cells, however, and much of our inference almost the RNA globe has come from experiments in which big pools of RNA molecules of random nucleotide sequences are generated in the laboratory. Those rare RNA molecules with a property specified by the experimenter are and so selected out and studied (Figure vi-97). Experiments of this blazon have created RNAs that tin catalyze a broad variety of biochemical reactions (Table six-iv), and suggest that the primary deviation betwixt protein enzymes and ribozymes lies in their maximum reaction speed, rather than in the diversity of the reactions that they tin can catalyze.

Figure 6-97
Beginning with a big pool of nucleic acrid molecules synthesized in the laboratory, those rare RNA molecules that possess a specified catalytic activity tin can exist isolated and studied. Although a specific case (that of an autophosphorylating (more...)
Table 6-four
Some Biochemical Reactions That Can Be Catalyzed by Ribozymes.
Like proteins, RNAs can undergo allosteric conformational changes, either in response to small molecules or to other RNAs. One artificially created ribozyme can exist in two entirely different conformations, each with a different catalytic activity (Figure 6-98). Moreover, the structure and function of the rRNAs in the ribosome alone have made it clear that RNA is an enormously versatile molecule. It is therefore like shooting fish in a barrel to imagine that an RNA world could attain a high level of biochemical sophistication.
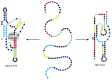
Figure vi-98
An RNA molecule that folds into two unlike ribozymes. This 88-nucleotide RNA, created in the laboratory, tin can fold into a ribozyme that carries out a cocky-ligation reaction (left) or a cocky-cleavage reaction (right). The ligation reaction forms a 2′,5′ (more than...)
Self-Replicating Molecules Undergo Natural Selection
The 3-dimensional folded structure of a polynucleotide affects its stability, its deportment on other molecules, and its power to replicate. Therefore, certain polynucleotides volition exist especially successful in any cocky-replicating mixture. Because errors inevitably occur in any copying process, new variant sequences of these polynucleotides will exist generated over time.
Sure catalytic activities would have had a cardinal importance in the early evolution of life. Consider in particular an RNA molecule that helps to catalyze the process of templated polymerization, taking whatever given RNA molecule every bit a template. (This ribozyme activity has been directly demonstrated in vitro, albeit in a rudimentary form that tin can simply synthesize moderate lengths of RNA.) Such a molecule, past interim on copies of itself, tin replicate. At the same time, it can promote the replication of other types of RNA molecules in its neighborhood (Figure 6-99). If some of these neighboring RNAs have catalytic actions that help the survival of RNA in other ways (catalyzing ribonucleotide product, for case), a set up of different types of RNA molecules, each specialized for a different activity, may evolve into a cooperative arrangement that replicates with unusually bully efficiency.
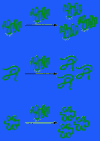
Figure half-dozen-99
A family of mutually supportive RNA molecules, one catalyzing the reproduction of the others.
I of the crucial events leading to the formation of effective self-replicating systems must have been the evolution of individual compartments. For example, a prepare of mutually benign RNAs (such as those of Effigy 6-99) could replicate themselves only if all the RNAs were to remain in the neighborhood of the RNA that is specialized for templated polymerization. Moreover, if these RNAs were gratis to diffuse amongst a big population of other RNA molecules, they could be co-opted by other replicating systems, which would then compete with the original RNA system for raw materials. Selection of a gear up of RNA molecules co-ordinate to the quality of the self-replicating systems they generated could not occur efficiently until some class of compartment evolved to contain them and thereby brand them available only to the RNA that had generated them. An early, rough form of compartmentalization may have been simple adsorption on surfaces or particles.
The demand for more sophisticated types of containment is easily fulfilled by a class of pocket-size molecules that has the simple physicochemical property of being amphipathic, that is, consisting of i part that is hydrophobic (water insoluble) and another office that is hydrophilic (water soluble). When such molecules are placed in water they aggregate, arranging their hydrophobic portions as much in contact with one some other as possible and their hydrophilic portions in contact with the water. Amphipathic molecules of appropriate shape spontaneously aggregate to grade bilayers, creating small closed vesicles whose aqueous contents are isolated from the external medium (Effigy 6-100). The phenomenon can exist demonstrated in a test tube by but mixing phospholipids and water together: under appropriate atmospheric condition, minor vesicles will form. All nowadays-mean solar day cells are surrounded by a plasma membraneconsisting of amphipathic molecules—mainly phospholipids—in this configuration; we hash out these molecules in detail in Chapter ten.
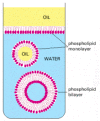
Figure six-100
Formation of membrane by phospholipids. Because these molecules accept hydrophilic heads and lipophilic tails, they align themselves at an oil/water interface with their heads in the water and their tails in the oil. In the water they associate to grade (more...)
Presumably, the offset membrane-divisional cells were formed past the spontaneous assembly of a set of amphipathic molecules, enclosing a cocky-replicating mixture of RNA (or pre-RNA) and other molecules. It is not articulate at what betoken in the evolution of biological catalysts this first occurred. In any case, once RNA molecules were sealed within a closed membrane, they could brainstorm to evolve in earnest every bit carriers of genetic instructions: they could exist selected not merely on the basis of their own structure, but also co-ordinate to their result on the other molecules in the same compartment. The nucleotide sequences of the RNA molecules could now exist expressed in the character of a unitary living cell.
How Did Protein Synthesis Evolve?
The molecular processes underlying protein synthesis in nowadays-day cells seem inextricably complex. Although nosotros empathise most of them, they do not make conceptual sense in the way that DNA transcription, Dna repair, and Deoxyribonucleic acid replication do. Information technology is especially difficult to imagine how protein synthesis evolved because it is now performed by a complex interlocking organisation of protein and RNA molecules; plainly the proteins could non have existed until an early on version of the translation apparatus was already in place. Although we tin only speculate on the origins of protein synthesis and the genetic code, several experimental approaches have provided possible scenarios.
In vitro RNA pick experiments of the blazon summarized previously in Figure 6-97 have produced RNA molecules that can bind tightly to amino acids. The nucleotide sequences of these RNAs often contain a unduly high frequency of codons for the amino acid that is recognized. For example, RNA molecules that demark selectively to arginine take a preponderance of Arg codons and those that bind tyrosine have a preponderance of Tyr codons. This correlation is not perfect for all the amino acids, and its interpretation is controversial, only information technology raises the possibility that a limited genetic lawmaking could take arisen from the directly association of amino acids with specific sequences of RNA, with RNAs serving equally a crude template to direct the non-random polymerization of a few different amino acids. In the RNA earth described previously, any RNA that helped guide the synthesis of a useful polypeptide would have a great advantage in the evolutionary struggle for survival.
In present-day cells, tRNA adaptors are used to match amino acids to codons, and proteins catalyze tRNA aminoacylation. Notwithstanding, ribozymes created in the laboratory can perform specific tRNA aminoacylation reactions, then it is plausible that tRNA-like adaptors could have arisen in an RNA world. This development would take made the matching of "mRNA" sequences to amino acids more efficient, and it perchance allowed an increase in the number of amino acids that could exist used in templated poly peptide synthesis.
Finally, the efficiency of early forms of protein synthesis would exist increased dramatically by the catalysis of peptide bail germination. This evolutionary development presents no conceptual problem since, every bit we have seen, this reaction is catalyzed by rRNA in present-day cells. One tin envision a crude peptidyl transferase ribozyme, which, over time, grew larger and caused the ability to position charged tRNAs accurately on RNA templates—leading eventually to the modern ribosome. Once poly peptide synthesis evolved, the transition to a protein-dominated world could continue, with proteins somewhen taking over the bulk of catalytic and structural tasks because of their greater versatility, with 20 rather than 4 different subunits.
All Present-mean solar day Cells Use DNA as Their Hereditary Material
The cells of the RNA world would presumably accept been much less complex and less efficient in reproducing themselves than even the simplest present-day cells, since catalysis by RNA molecules is less efficient than that by proteins. They would have consisted of picayune more than a simple membrane enclosing a set of self-replicating molecules and a few other components required to provide the materials and energy for their replication. If the evolutionary speculations virtually RNA outlined above are right, these early on cells would also have differed fundamentally from the cells nosotros know today in having their hereditary data stored in RNA rather than in DNA (Effigy 6-101).

Figure 6-101
The hypothesis that RNA preceded Dna and proteins in evolution. In the earliest cells, pre-RNA molecules would take had combined genetic, structural, and catalytic functions and these functions would have gradually been replaced by RNA. In present-day (more...)
Bear witness that RNA arose earlier DNA in development can be found in the chemical differences between them. Ribose, like glucose and other simple carbohydrates, can be formed from formaldehyde (HCHO), a simple chemic which is readily produced in laboratory experiments that endeavor to simulate weather condition on the primitive Globe. The carbohydrate deoxyribose is harder to brand, and in present-day cells information technology is produced from ribose in a reaction catalyzed by a protein enzyme, suggesting that ribose predates deoxyribose in cells. Presumably, DNA appeared on the scene later, but then proved more suitable than RNA as a permanent repository of genetic data. In particular, the deoxyribose in its sugar-phosphate courage makes chains of DNA chemically more stable than chains of RNA, and so that much greater lengths of DNA can be maintained without breakage.
The other differences between RNA and DNA—the double-helical construction of Dna and the use of thymine rather than uracil—farther enhance DNA stability by making the many unavoidable accidents that occur to the molecule much easier to repair, as discussed in detail in Chapter five (meet pp. 269–272).
Summary
From our knowledge of present-solar day organisms and the molecules they incorporate, it seems likely that the evolution of the directly autocatalytic mechanisms fundamental to living systems began with the evolution of families of molecules that could catalyze their own replication. With fourth dimension, a family of cooperating RNA catalysts probably developed the ability to straight synthesis of polypeptides. Dna is likely to have been a late addition: as the accumulation of additional protein catalysts immune more than efficient and complex cells to evolve, the DNA double helix replaced RNA as a more stable molecule for storing the increased amounts of genetic information required past such cells.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26876/
0 Response to "Which of the Following Can Both Catalyze Chemical Reactions and Carry Information to Copy Itself?"
Post a Comment